In chemical process industries, we can find the use of steam distillation method for many services. Mainly, we use this technique where we want to distil the components form high boiling point mixtures or altogether non-volatile salt solutions. For example, recovery of 2-chloropyridine from neutralized solution containing pyridine, NaCl and water.
Moreover, steam distillation become preferable when the material decomposes at high boiling point temperatures. This includes extracting some natural products for example, to extract eucalyptus oil from eucalyptus, citrus oils from lemon or orange peel, and to extract oils used in perfumes from various plant materials.
Therefore, to overcome these types of problems we can use steam distillation in place of direct distillation method. This way we can perform distillation at low temperature, which is close to the boiling point of water. However, steam distillation is more preferable when solubility of water in organic chemicals is negligible or very low.
Table of Contents
What is a Steam Distillation Process?
So, first let us understand about the steam distillation process. In this process we pass steam directly in the liquid mixture through a sparger, which is filled in a vessel. Moreover, in some cases we can supply heat indirectly also using reboiler. Here, mixture contains sufficient water quantity required for steam distillation. This process can be batch or continuous, and depends upon the production capacities. For high production capacities continuous process is more economical and operational friendly. This steam distillation is similar to steam stripping process.
Batch Steam Distillation
So, the batch distillation we use where production capacity is low or product requirement is not consistent. This can be mainly in the case of fine chemicals and specialty chemicals manufacturing. To understand the batch steam distillation or differential distillation process, we can refer below figure.
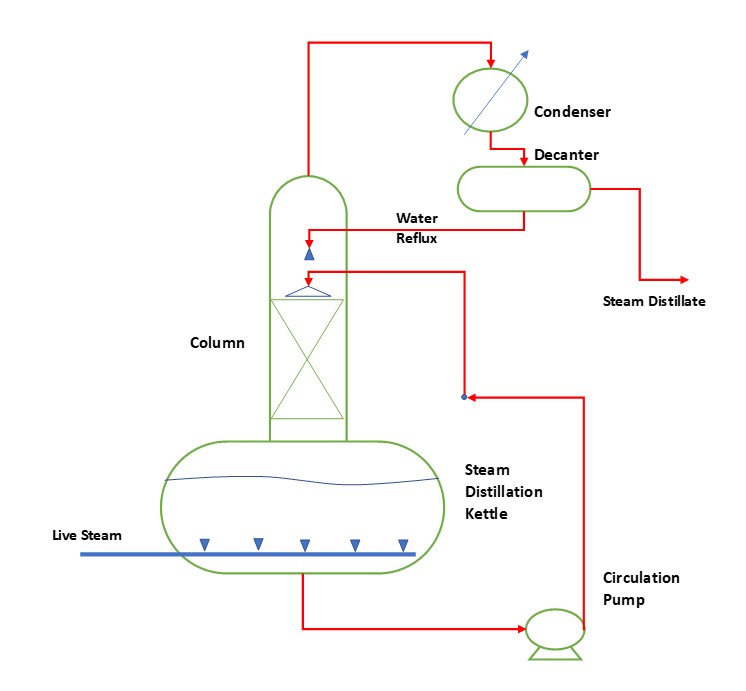
We charge crude product into the kettle and keep it in circulation mode using a circulation pump. If there is no water in the mass then we add water in the kettle. A small column filled with packing will enhance the contact surface area between steam and high volatile chemicals. However, in case of fouling or chocking nature of feed, we should use trays or random packing inside the column. In kettle we feed live steam through a sparger in crude mixture liquid pool. And, from column top vapor containing steam and low volatile chemicals goes into the condenser. This condensed liquid is steam distillate and goes for further separation of product.
Since, water and organics are not soluble or slightly soluble in each other. Here we can use a decanter and separate the organic mixture from water by layer separation. From decanter water layer will go for effluent treatment or refluxed back. While, organic layer goes for further distillation to get pure product.
Continuous Steam Distillation
We use continuous process to meet the high production capacity requirements. We call this process as steam stripping also, where we remove volatile organic chemicals from the non-volatile or very high boiling components. This feed mixture contains water, salt and organics. To understand continuous steam distillation process, you can refer below figure.
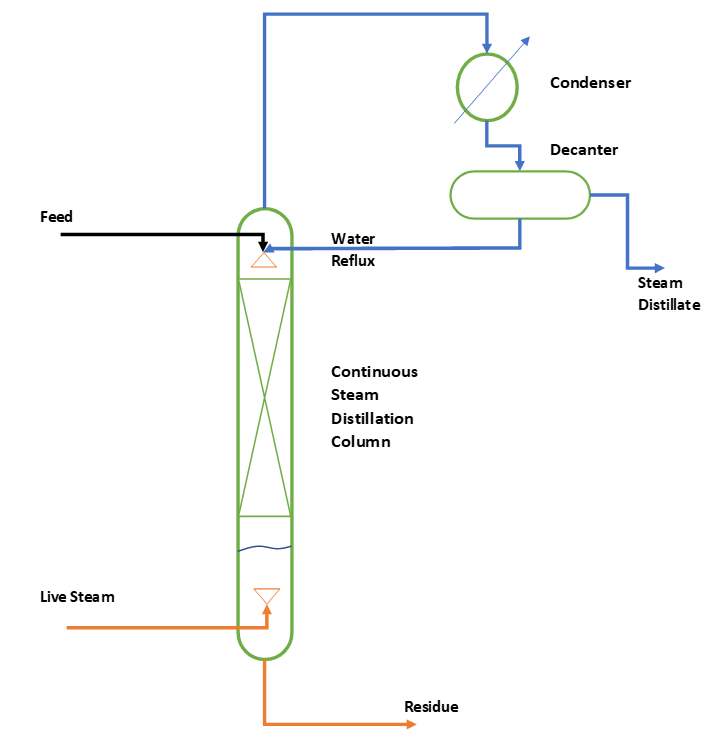
So, we can see for continuous steam distillation we have a packed tray type distillation column. For fouling services structured packing is not recommended, in such cases we should use trays or random packing.
We introduce feed from top side of the column, while live steam is introduced from bottom side. Rising steam contact with downward moving column feed and volatile chemical is stripped off from the mixture. From column top vapor containing water and organic mixture goes into condenser. Subsequently, steam distillate goes for further processing for product separation and purification. When organic compound is immiscible in water, we use decanter to separate organic layer from water layer. From here, organic layer goes for further distillation to get the pure product. While, aqueous layer goes for reflux or effluent treatment unit, which depends on the process.
However, sometimes there can be cases when organic and water is miscible and forms no layers. In such cases we need to use some suitable solvent to extract organic from the steam distillate. After extraction, organic layer goes for solvent and pure product recoveries and aqueous layer is raffinate and transferred to effluent treatment facility.
We use reflux to enrich the organic contents in steam distillate and to maintain the top steam distillation temperature. The bottom stream from this column goes to next level of processing, which is mostly incineration or land filling.
In below section we will discuss the principle behind the steam distillation process.
Theory of Steam Distillation
So, to understand the fundamental behind steam distillation, let us consider the component ‘A’ which we want to recover from a mixture. In this mixture other components are very high boiling or non-volatile salts. And, we designate subscript ‘B’ to the steam.
The theory of direct steam distillation is based on partial pressures of the immiscible organic components and the presence of direct open steam in the system. This system may consist of the organic immiscible plus steam. As we know each liquid exerts its own vapor pressure independent of the other. Thus, the total pressure of the system is the sum of the individual vapor pressures of the two liquids (assuming the liquids do not dissolve in each other, like water & toluene).
So, for steam ‘B’ (saturated vapor, no liquid) distillation with one organic component ‘A’ (liquid).
For given system pressure P and steam distillation temperature T, we can estimate the partial pressure of the component ‘A’, which is equal to its vapor pressure at temperature T. So, total pressure of the system will be equal to the sum of partial pressure of steam and component ‘A’.
Total pressure will be, P = PA + PB
Moreover, we can estimate the mol fraction of each components in column vapor as below.
Mol fraction of steam in vapor, yB = PB/P and for compound ‘A’ is, yA = PA/P, so mass of each components in vapor will be.
(mA/MA) = yA and (mB/MB) = yB, where m is mass and M is molecular weight.
So, from above relations we can get,
yA/yB = PA/PB = PA/(P – PA) = (mA/MA)/ (mB/MB)
Now, let us consider a mixture to do the process calculations as below.
Steam Distillation of Toluene from High Boiling Mixture
We have to design a steam distillation system to separate the toluene from a high boiling temperature mixture (i.e. boiling temperature > 220 0C). The design basis for system is as below:
Total quantity : 5000 kgs
Toluene Content : 50 wt%
Non-Volatile Content : 50 wt%
Steam Pressure : 3 bar @ saturated conditions
This is a batch distillation set up operating at atmospheric pressure. Steam latent heat = 516 kcal/kg and temperature is 133 0C. We are using steam distillation because at high distillation temperature non-volatile material has polymerization tendency. Therefore, we need to keep the boiling point <140 0C.
Steam Distillation Temperature Estimation
So, the intersection of (P – PB) and PA curves gives the temperature of distillation, which is 84.35 0C. This we can see in below figure also.
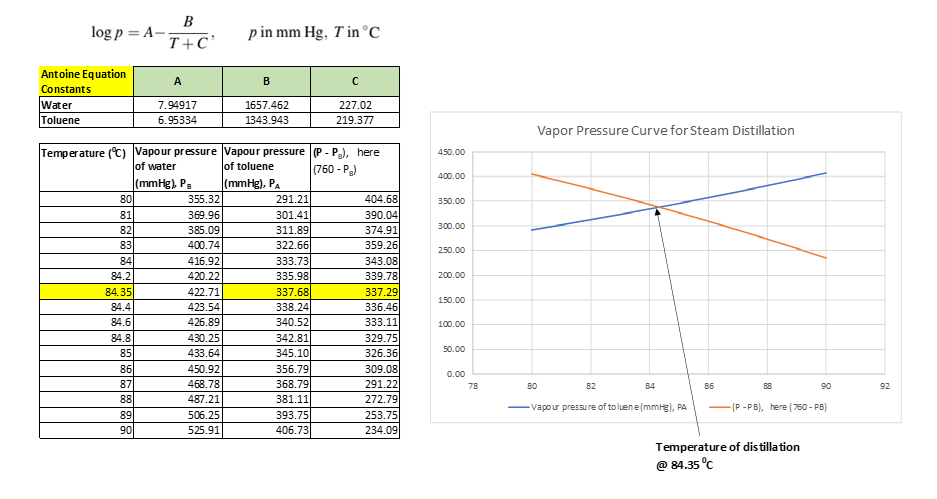
In above figure we have calculated the vapor pressure of water & toluene at temperature range between 80 – 90 0C, using Antoine equation. Subsequently, we estimated the value of (P – PB) and plot the data (P – PB) and PA v/s temperature. Both these curves intersect each other, which will be temperature for steam distillation at P = 101.3 kPa pressure or atmospheric pressure.
Moreover, as we discussed above the molar ratio of water to toluene material is given by (P − PA)/PA. Therefore, the mass-ratio of water to toluene in the vapour will be:
(mA/MA)/ (mB/MB) = PA/(P – PA)
(mB/mA) = [(P – PA)/ PA]*(MB/MA)
Here,
MA = 92, MB = 18, P = 101.3 kPa, PA = 337 mmHg = 44.8 kPa
(mB/mA) = [(101.3 – 44.8)/(44.8)]*(18/92) = 0.247 kg/kg
This means (1/0.247 = 4.05), in other words 4.05 parts of toluene to 1 part of water. Hence, steam going with toluene considering 100% recovery should be, W1 = 0.247*2500 = 617.5 kgs.
So, heat value of 617.7 kgs steam, Q1 = 617.7*516 = 318,733 kcal
Total heat requirement to recover toluene will be as below, considering initial charge temperature is 35 0C, latent heat of vaporization for toluene is 84 kcal/kg and heat capacity of mixture is 0.35 kcal/kg0C.
Q = 0.35*5000*(84 .4 – 35) + 2500*84 = 296,450 kcal
We have considered rest of the mixture as non-volatile high boing temperature mixture; therefore, we have not considered the heat load for reflux.
Conclusion
Finally, we discussed how can we use steam distillation to separate out temperature sensitive compounds or volatile organics from non-volatile mixtures. During batch process the composition of compound in bottom distillation vessel changes. As we move towards the end of distillation the non-volatile material composition is high, which increases the bottom temperature. In result, there will be no water in bottom and the saturation pressure of the steam will be higher and separation will be difficult. Therefore, the steam distillation efficiency remains in the range from 60% to 70% for many organics. However, we can achieve better efficiencies with good design of steam sparger, which can be around 90% to 95%.
We can do steam distillation using a jacketed agitated vessel, charge the mixture and add water quantity as we estimated above (i.e., in case of water and toluene, it is 1.0 kg water per 4.05 kg of toluene). Heat the mixture and condense the vapor, after decantation we can separate toluene and water layers in different collection vessels.
Thanks for reading and I welcome your feedbacks.
