In chemical engineering, model equation enable us to understand the behavior of the process. Therefore, using mathematical models we can run the process virtually and get optimum operating process parameters. Afterwards, we can use these optimized parameters for real life process.
Table of Contents
System Description
We have an agitated reactor having volume V, in which two liquid streams are incoming. Both liquid streams are two different chemicals A and B. When these two chemicals contact with each other into the reactor, they react and forms a new chemical D. The volumetric flow rates of chemical A and B are qAf and qBf respectively. The concentration of chemical A & B in their feed are CAf and CBf. The reactor is well mixed. Exit stream volumetric flow rate is q and concentration of various chemicals are CA, CB and CD. The schematic figure of system is as below
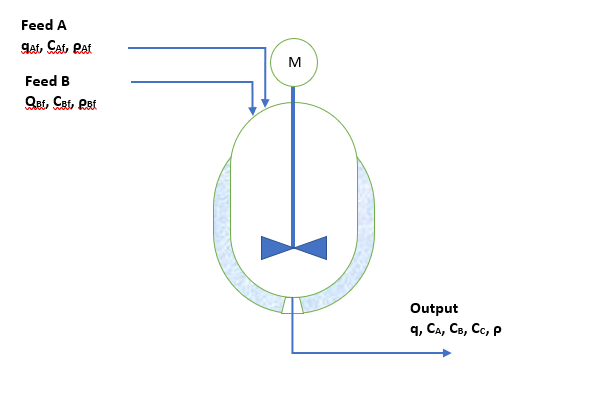
Apply the law of mass conservation between a time span t1 and t2 which is very small-time interval (i.e., t2-t1=∆t)
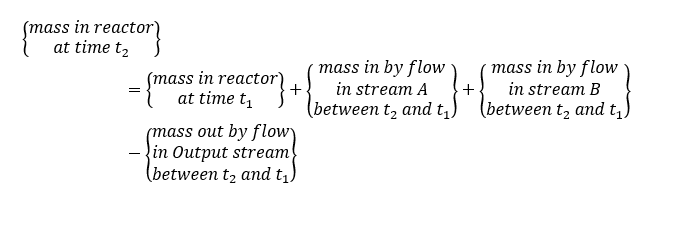
Total Mass Balance
So, we can write the total mass balance for above control volume system as below
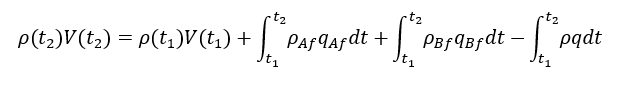
Integration of above equation will give
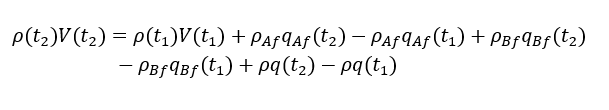
considering t1 = t and t2 = t + ∆t, assuming ρ & q constant and t2-t1=∆t →0
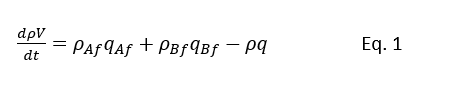
Component Mass Balance
Mass balance for Chemical species A will be as below for the control volume V,
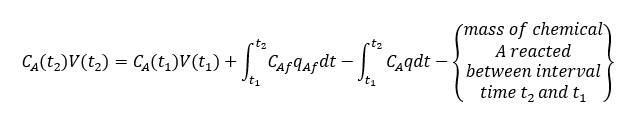
For our system let us assume the reaction between A and B is as below
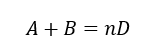
Rate of consumption for chemical A in reaction = rAV
Amount of chemical A converted because of reaction between time t2 and t1 = rAVdt
So, component mass balance for species A will be, putting consumption term in above equation
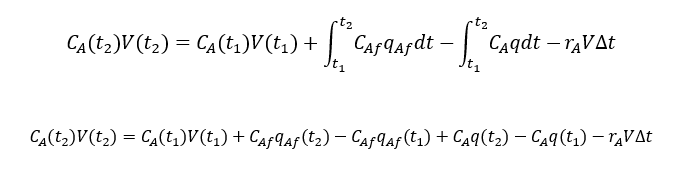
considering t1 = t and t2 = t + ∆t, assuming ρ & q constant and t2-t1=∆t →0
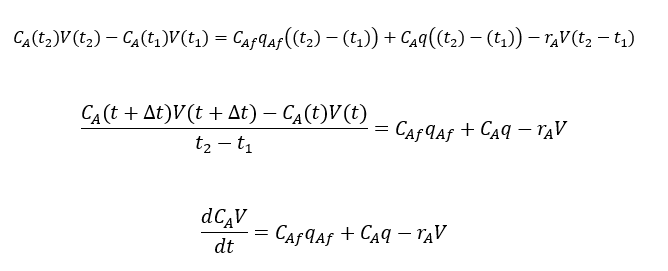
Similarly, we can write component balance for chemical B and D also
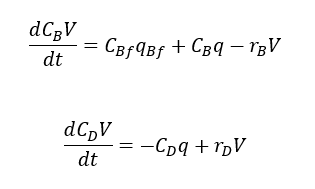
From reaction we understand that, one mol of A reacts with one mole of B to form n mols of D. So, we can write the relationship between rate of reactions
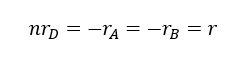
Then component balance will become as below
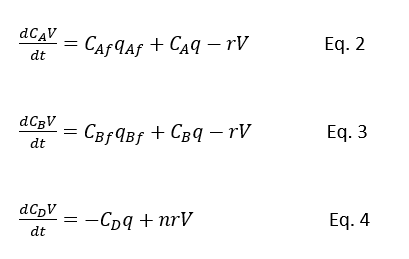
Equations are Eq.1 to Eq.4 are the basic model equations for a well-mixed agitated reactor in which the liquid phase reaction between A and B to form D is taking place.
Model for Batch Reactor
For modeling let us take following assumptions
Densities of all streams are equal (i.e., ρ = ρAf = ρBf )
since this is a batch reactor, so there is no in or out flow from this (qAf = qBf = q = 0) and volume V is constant, Equation for 2 to 4 will reduce to

Equation from Eq.5 to Eq.7 are basic model equation for batch reactor system.
If initial total concentration of A and B in reactor is CA0 and CB0 respectively than, M = CA0 + CB0 . Hence CB = (M – CA)
For our system let us assume the reaction between A and B is as below
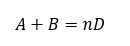
Assume this is elementary reaction than rate of reaction will as below
r = kCACB
The Eq.5 will be reduced as below
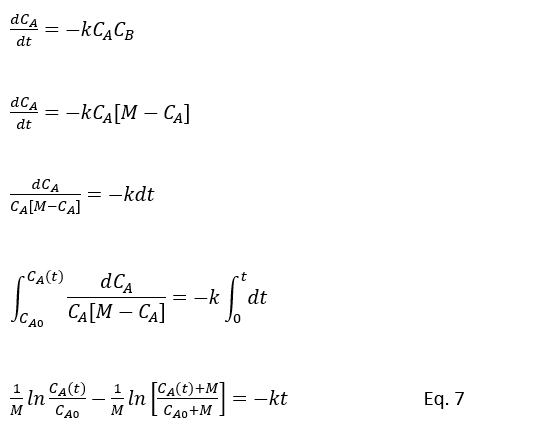
Eq.7 will reduce for CA in a batch reactor with second order rection A + B → nD, for equal (M=0) starting concentration
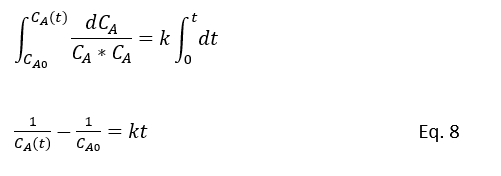
Sample Problem
The hydrolysis of acetic anhydride in excess water to form acetic acid (CH3CO)2O + H2O → 2CH3COOH and the rate of reaction at 15 0C can be expressed as r = kCA, k=0.0567 min-1. This reaction study takes place in a batch reactor and initial concentration CA0 = 100 lb of acetic anhydride. So the time taken to convert 90 lb of anhydride can be estimated by model equation Eq. 5.
CA0 = 100 lb, CA = 90 lb and k = 0.0567 min-1
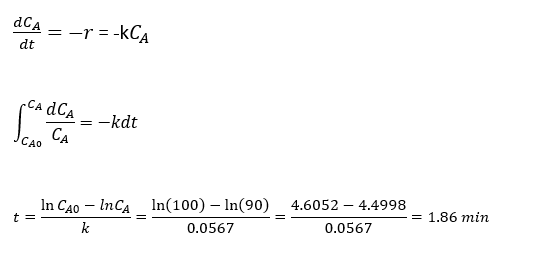
Reference: Introduction to Chemical Engineering Analysis, T.W.E. Russell & M.M. Denn.

