In reality all chemical reactions are reversible in nature. Moreover, final conversion of raw materials depends on the ratio of forward and backward reaction rates. During the reaction, at equilibrium state concentrations of reactants and products don’t change. Also, for some reactions when forward reaction is too high in comparison with reverse reaction rate and equilibrium lies > 99.9%, in such case we can consider reaction is complete. In these type of reactions, we can omit reverse reaction. We can workout these information for a reaction using chemical thermodynamics fundamentals.
To understand the equilibrium constant let us consider an example, where chemicals A & B are reactants and R & S are products.
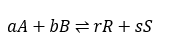
The Equilibrium constant for above reaction cab be given as below
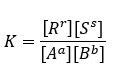
In above equation [A], [B], [R] and [S] are concentrations of respective components in mole/liter. A large value of K tells us the concentrations of [R] & [S] are very high and conversion for reaction is also high.
Based on the heat of reaction we can categorize chemical reactions in two classes
Table of Contents
Exothermic Reactions
During any reaction some bond are breaks and new bonds are formed. When weaker bonds are breaks and stronger bonds forms then heat is released from the reaction and we call It is an exothermic reaction. In such reactions we need to remove heat continuously from the reactor to maintain the required reaction temperature. If we don’t remove heat from the reactor, reactor temperature will keep on increasing adiabatically and will lead to run away. Reaction run away can cause huge loss to a company in terms of money and human lives. Example of exothermic reactions are neutralization, oxidation of ethanol, chlorination of methane, combustion etc.
Endothermic Reactions
In case of endothermic reactions stronger bonds break and weaker bonds form. To sustain these type of reaction we need to supply heat to the reactor continuously. To achieve desired conversion it is very important to maintain the reactor temperature. If we fail to supply the heat of reaction into the reactor, reaction will cool down and production will stop. We can see the examples for endothermic reactions are thermal cracking of acetic acid, electrolysis of water, urea from ammonia carbamate, etc.
Chemical Thermodynamics
We can check the feasibility of a chemical reaction using chemical thermodynamic whether it is possible or not. It is used to estimate equilibrium conversion and effect of temperature and pressure on the degree of conversion.
Equilibrium of a System
When a system is in equilibrium, it is considered as isolated system and there is no spontaneous change in the it’s parameters. In equilibrium state properties of the system are uniform and each property can take only one value.
Chemical Potential
In chemical thermodynamics, chemical potential of a species is energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition.
Chemical potential µi of component i is as below
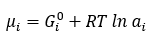
Where, Gi0 = standard Gibbs free energy of component i
ai = fi/fi0 = activity of component i
fi = fugacity of component i
fi0 = standard state fugacity of component i
For a chemical reaction at equilibrium Ʃνiµi = 0, here νi is stoichiometric coefficient & µi is chemical potential of component i in the chemical reaction. For our reaction stochiometric coefficient is r, s for component R & S and for A & B it is a, b respectively.
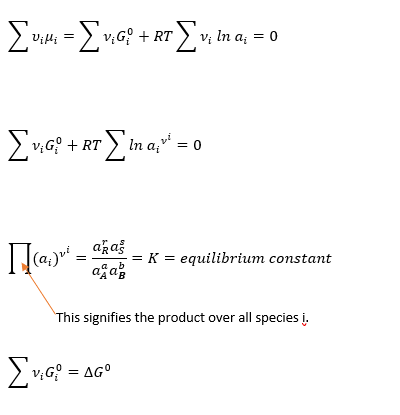
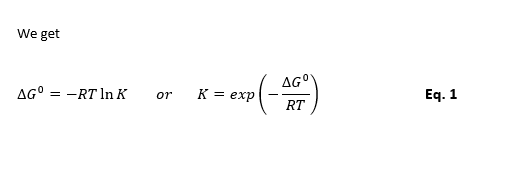
Effect of Temperature on Equilibrium Constant
For a reaction dependency of Gibbs free energy on temperature at constant pressure is as below
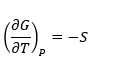
And the change in the standard state Gibbs free energy is as follows
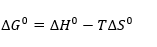
From above to equation we get another equation is the Gibbs-Helmboltz equation
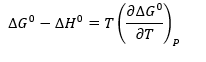
As we have, ∆G0 = – RT ln K, so we can rearrange Gibbs-Helmboltz equation to a new equation is Vont Hoff equation.
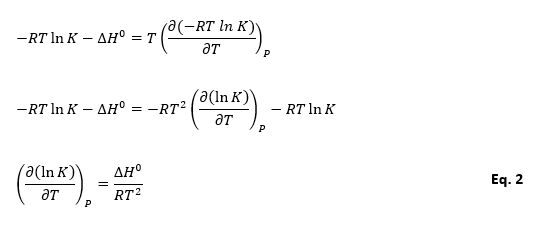
Above equation is Vant Hoff equation and this gives the effect of temperature on the chemical reactions. For an exothermic reaction (∆H0 < 0) and equilibrium constant decreases with increase in temperature. While for endothermic reactions (∆H0 > 0) and equilibrium constant increases with the increase in temperature.
Estimation of Equilibrium Constant as any Temperature
Integration of Vant Hoff equation gives

Effect of Pressure on Conversion
- During the reaction if there is no change in the moles, in this case pressure will not affect the reaction.
- When numbers of moles decrease during the reaction, the increase in pressure will increase the reaction conversion.
- The case when moles increases in reaction then, conversion of raw materials will increase with decrease in reactor pressure.
Effect of Inert Gas addition on Conversion
Inert addition will make no impact on the conversion if there is no change in the moles after reaction. Conversion will increase with addition of inert gas when there is increase in number of moles in reaction. For the reactions where moles increase during reaction, inert addition will make a positive impact on the input material conversion.
Effect of Excess Input Material on Conversion
Reaction conversion will increase with the increase of all input material quantity except the limiting reactant. This will shift the reaction in forward direction.
Product Material effect on the Reaction Conversion
Raw material conversion will decrease if there is there are some products are present.
Conclusion
Reactor is the most important part of a chemical industry. For reactor designing it is critical to understand the effect of temperature, pressure and reactants concentration. Because, any miscalculation of a reactor can lead to financial and human lives loss. Therefore, chemical thermodynamics enables to model the reactor, which help a designer to design a safe and efficient reactor.
- Reference:
- Theory and Problems of Thermodynamics – Y.V.C. Rao

