In chemical process industries we can face two types of mixture. First, where purity of more volatile components by simple distillation does not take place after a certain concentration. And, in second category it takes place so slowly that an uneconomic number of plates is required. So, to separate these types of mixtures we use azeotropic or extractive distillation method.
You can find simple example of an azeotrope is distillation of ethanol and water mixture. Where the concentration of the alcohol steadily increases until it reaches 96% by mass. And, when the composition of the vapour equals that of the liquid, and no further enrichment occurs. We call this mixture an azeotrope, and we cannot separate it by simple distillation.
Other than azeotropic mixture, second problem occurs where the relative volatility of a binary mixture is very low. Or in other words we can say their boiling point difference is very low, in such cases relative volatility of the mixture is close to 1.0. An example of this type is the separation of n-heptane from methyl cyclohexane in which the relative volatility is only 1.08. And, in this case large number of plates are required to achieve separation. Moreover, continuous distillation of this mixture to give nearly pure products will require very high reflux ratios. Therefore, we need a tower of large cross-section containing many trays and high steam consumption to meet the high reflux ratios.
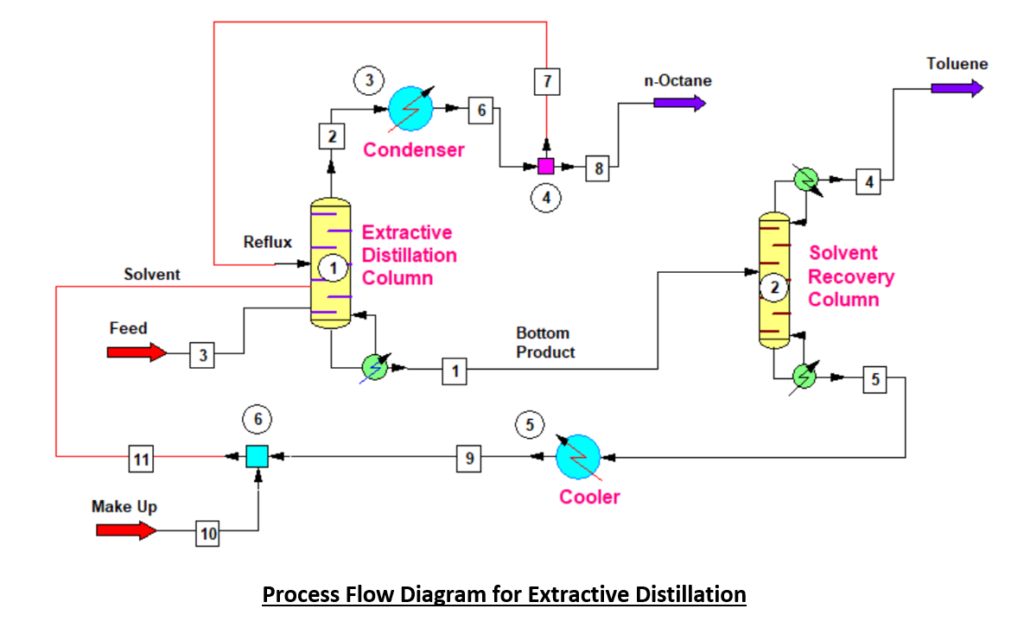
In this article we will go through extractive distillation process. And, also discuss the process simulation results to separate the mixture of iso-octane and toluene using phenol solvent.
Table of Contents
Concept of Extractive Distillation Method
So, extractive distillation is a method of rectification of close boiling or constant boiling mixture which is similar in purpose to azeotropic distillation technique. To a binary mixture which is difficult or impossible to separate by simple distillation, we add a third component, termed as a solvent. This component alters the relative volatility of the original constituents, thus permitting the separation. Moreover, this added solvent has low volatility and has nil or negligible concentration in distillate. In other words, total solvent comes out as it is with the bottom product of distillation column. However, in some cases solvent comes out with distillate and has negligible or nil concentration in bottom product.
Extractive distillation process depends upon the difference from ideality between the solvent and the components of the mixture. So, in our example both toluene and iso-octane separately make non-ideal liquid solutions with the solvent (i.e., phenol). The degree of non-ideality of solution of phenol with iso-octane is greater than toluene. Therefore, in presence of phenol the toluene and iso-octane themselves behave as a non-ideal mixture, and their relative volatility becomes high. Which makes distillation easier.
Extractive Distillation Process for Iso-octane and Toluene Separation
To understand the process, you can refer the below flowsheet. We feed iso-octane and toluene mixture into the extractive distillation column. Moreover, phenol as a solvent enter into the distillation column from the top. So, phenol extract the toluene from upcoming vapour and we recover pure iso-octane as a distillate. While, toluene and phenol come out from bottom of the extractive distillation column.
Subsequently, residue from extractive distillation column enters into the solvent recovery column. Where, phenol is separated from toluene and recycled back to the first column as a solvent. And, toluene is recovered from the bottom of the solvent recovery column.
So, for the lower cost of separation, suitable solvent selection is very important. During solvent selection we must consider solvent selectivity, volatility, ease of separation from the top and bottom products, and the cost. Moreover, it is important to note that the solvent must not form an azeotrope with any of the components. When we talk about solvent selectivity, it means how much tendency it has coming out with top and bottom product. We must select a solvent which has no or negligible tendency of coming out with distillate or bottom. In other words, one of the product streams from extractive distillation column must be free from solvent.
Simulation Results for Extractive Distillation for iso-Octane and Toluene
In this simulation we are feeding a mixture of iso-Octane and Toluene. Feed flow rate is 1000 kg/h and contains 40% toluene and 60% iso-Octane or 2,2,4-Trimethylpentane by wt. %. This is an atmospheric pressure distillation process. The boiling point of iso-octane at atmospheric pressure is 99 0C, while toluene normal boiling point is 110.6 0C. Here, iso-octane in this mixture is the more volatile, although the separation is obviously difficult because of low relative volatility.
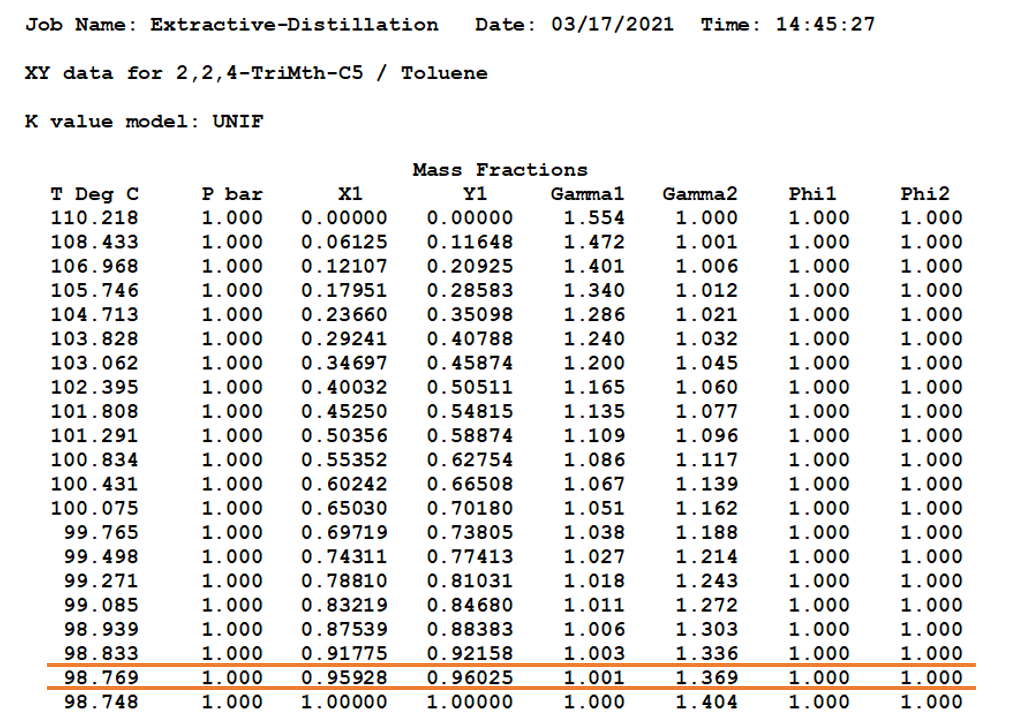
Equilibrium Data for iso-Octane & Toluene Mixture
This we can see in below x, y diagram & data also, the iso-octane purification is very difficult after 85 wt.% and almost impossible beyond 96 wt.%. Where it is an azeotrope at 98.77 0C, which is highlighted between red lines.
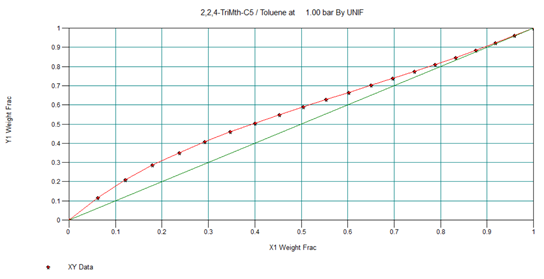
So, to separate this mixture we selected extractive distillation method and used phenol as a solvent. In the presence of phenol, boiling point 181.7 0C, the relative volatility of iso-octane increases. And, the separation of iso-octane from toluene is relatively easy.
Material Balance Table for Extractive Distillation Process
Below is material balance table which is the result of process simulation on Chemcad. In this table to identify the stream you need to refer process flow diagram as given above. Here you can see stream-8, the purity of iso-octane is 99.17 wt.%, which is the distillate of extractive distillation column. Also, the second component toluene is the distillate of solvent recovery column. This is the stream-4 and toluene purity is 99.99 wt.%.
Moreover, based on simulation results phenol which we are using as a solvent in extractive distillation column has flow rate 2881 kg/h. This column has 30 numbers theoretical trays, and we are feeding solvent at 3rd stage from the top. While, feed is entering at 11th stage from top and reflux stream is at 1st stage of the column. The reflux ratio for this column is around 1 : 6 (i.e., exactly 1 : 5.8 from below data), which we can see below stream-7 has flow rate 3500 kg/h. However, we can consider reflux ratio for this separation 1 : 6.
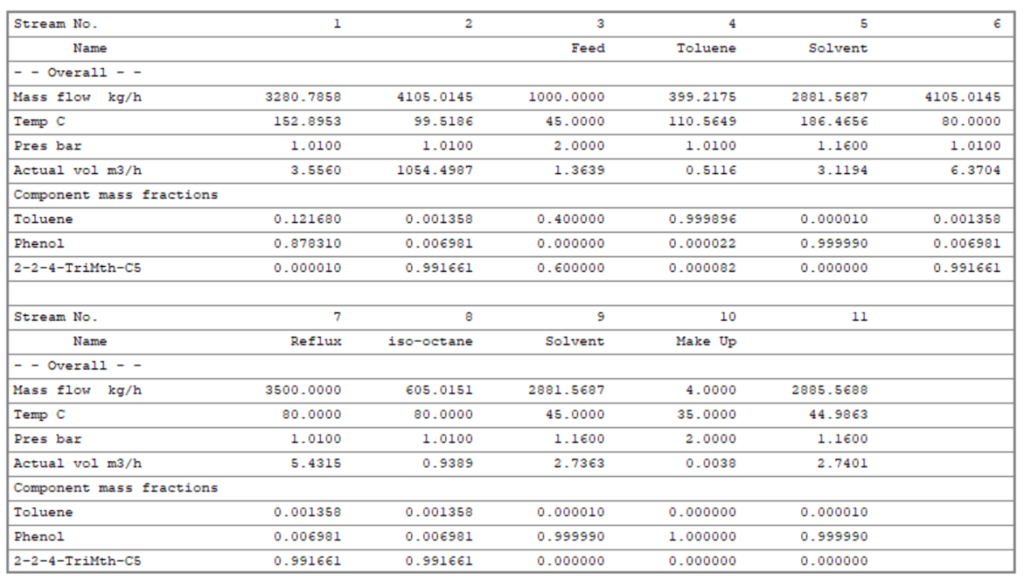
In solvent recovery column I have considered 20 numbers theoretical stages and fed is entering at 10th stage. Moreover, this column is operating at 1 : 5 reflux ratio.
I have selected UNIFAC thermodynamic package for this simulation.
Conclusion
So, you can use this process to separate the close boiling components. And, process design for columns, condensers, reboilers and other exchangers can be carried out as we do in case of simple distillation column.
From the operational ease extractive distillation method is usually more desirable than azeotropic distillation since no large quantities of solvent have to be vaporized. In addition, a greater choice of added component is possible since the process is not dependent upon the accident of azeotrope formation. Also, for the efficient and safe operation, we need to provide proper instrumentation and controls. To understand it you can refer to my article Instrumentation & Controls in Distillation Column.
Thanks for your reading looking forward for your feedbacks.
